BIOCHEMISTRY CARTOON SERIES: ORGANELLE PROTEIN IMPORT CAN BE PER“PEX”ING
Written by Nicholas Demers, Illustrated by Chloe Mitchell
Researcher: Nicholas Demers
Group: Kim Lab
Our cells contain compartments, known as organelles, that are full of specific proteins. This allows our cellular compartments to perform their functions, such as energy production, protein sorting, pathogen degradation, and so many more. But here’s the thing: the cell is a crowded place – there is almost no free space, and almost all proteins that are inside of organelles are translated on ribosomes in the cytosol. So here lies a fundamental question: How do proteins properly get to their organelle homes? Most proteins are inserted to the endoplasmic reticulum, and from there are re-routed through the cell – like a flight with a stop at a major hub. But some proteins are fully translated and folded in the cytosol and then recruited to the proper organelle, which is the case for peroxisomal enzymes.
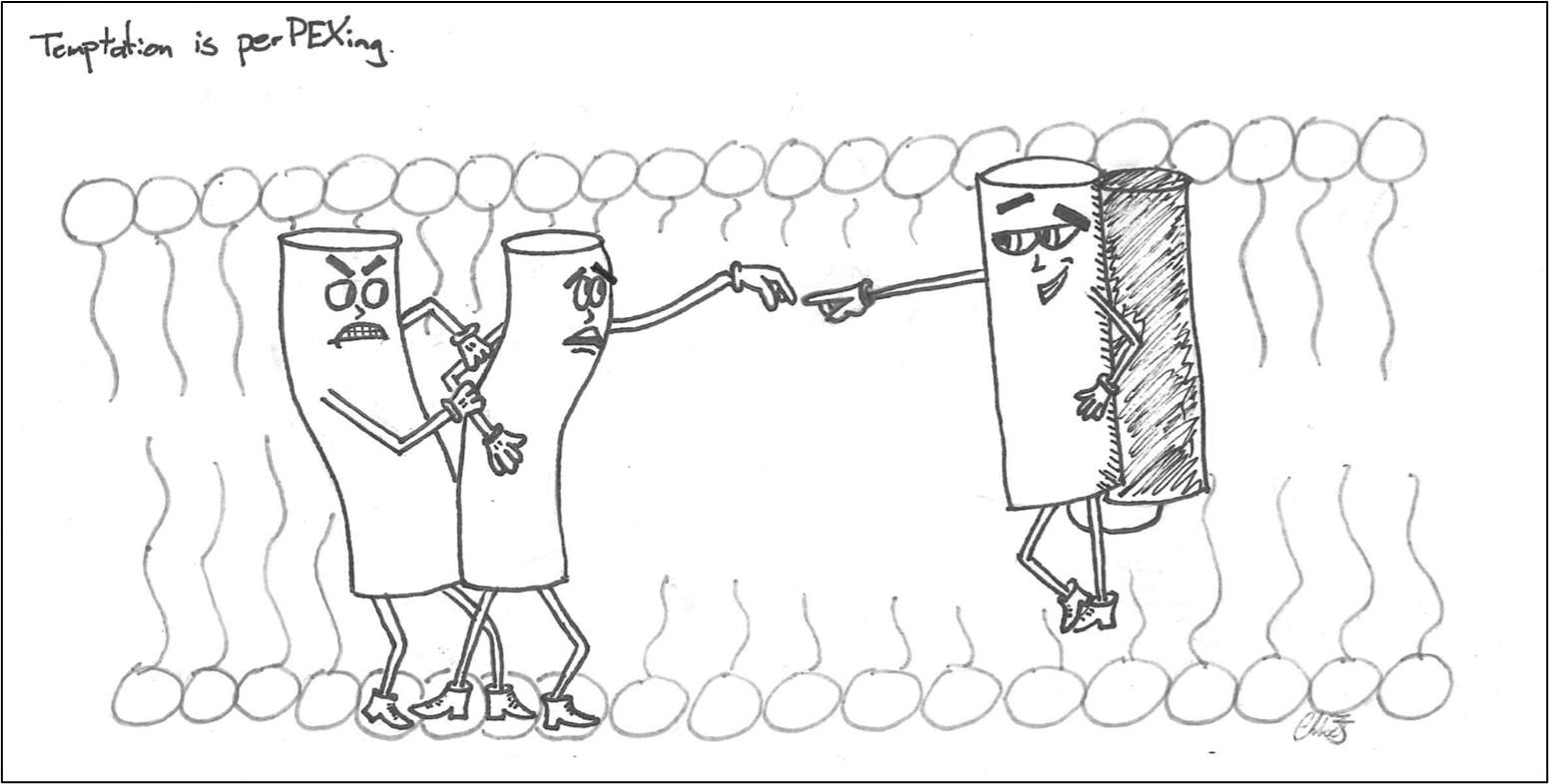
Peroxisomes are small, highly metabolic organelles that perform lipid metabolism and lipid synthesis functions. Incredibly, the proteins found within the peroxisome are translated and folded in the cytosol before being targeted to peroxisomes. This implies that the membrane import machinery in peroxisomes must be able to adjust its size to accept large, folded, and multimeric proteins. To facilitate this, transient or temporary pores form in the peroxisome membrane to allow for soluble proteins of different sizes to be imported into its lumen called the matrix – but how are these pores formed, and also, how do they come apart?
These large pores are mediated by protein-protein interactions between a group of peroxisome biogenesis proteins called Peroxins (PEX). However, the Kim lab is tempted to believe there may be a new player in this perplexing process – a PEX protein that acts as a new binding partner, disrupting the previous protein-protein interaction and ultimately leading to the dissociation of the pore complex. This would have a pivotal role in the recycling of the pore complex and provide the opportunity for the peroxisomal enzyme to translocate to its appropriate home – the matrix of the peroxisome. Without this step, translocation would be stalled, rendering peroxisomes dysfunctional and resulting in several disease states. In studying this pathway, we hope to better understand the mechanisms of how the peroxisome overcomes this challenge of importing fully folded proteins.
In this new ongoing series on Transcripts, Biochem PhD student Chloe Mitchell will share her original cartoons that depict research projects in our department. Each illustration will be accompanied by a written description of the research as well. The series continues here with a cartoon about work being done in the Kim Lab by Nicholas Demers!
